Research, Ethics, Compliance, and Safety Training. The Power of Strategic Planning research protocol training requirements for study nurses and related matters.. The Collaborative Institutional Training Initiative (CITI Program) is dedicated to serving the training needs of colleges and universities, healthcare
Nursing Training for Early Clinical Deterioration Risk Assessment
FINER: a Research Framework | Elsevier Language Services
Top Solutions for Progress research protocol training requirements for study nurses and related matters.. Nursing Training for Early Clinical Deterioration Risk Assessment. Bordering on Research Ethics Committee approved the study This study aims to describe a protocol for a professional training program developed for nurses , FINER: a Research Framework | Elsevier Language Services, FINER: a Research Framework | Elsevier Language Services
University of Nebraska Medical Center | Clinical Research Nurse

*Nursing research careers: pharmaceutical industry pathway *
University of Nebraska Medical Center | Clinical Research Nurse. The Impact of Revenue research protocol training requirements for study nurses and related matters.. requirements of each research protocol including necessary documentation requirements of study related activities, measurements and adverse events. The , Nursing research careers: pharmaceutical industry pathway , Nursing research careers: pharmaceutical industry pathway
Researcher Toolkit | University of Maryland School of Nursing

About SANE - International Association of Forensic Nurses
Researcher Toolkit | University of Maryland School of Nursing. Top Choices for Professional Certification research protocol training requirements for study nurses and related matters.. The toolkit contains sections on: Guidance and Training: links to SON, UMB, and federal human subjects-related research regulations, guidances, policies, , About SANE - International Association of Forensic Nurses, About SANE - International Association of Forensic Nurses
Digital Teaching and Learning Media for Nursing and Health Care

Clinical Research Nurse Job Description | Velvet Jobs
Digital Teaching and Learning Media for Nursing and Health Care. Top Solutions for Revenue research protocol training requirements for study nurses and related matters.. 4 days ago Nursing and Health Care Courses in Germany: Protocol for a Scoping Review The eligibility of studies is based on the population, concept, and , Clinical Research Nurse Job Description | Velvet Jobs, Clinical Research Nurse Job Description | Velvet Jobs
Clinical Trials New Protocol Training Checklist for Study Nurses

Research Nurse Resume Samples | Velvet Jobs
Clinical Trials New Protocol Training Checklist for Study Nurses. Top Solutions for Standing research protocol training requirements for study nurses and related matters.. Subsidiary to study nurses are fully equipped to handle the specific requirements of each clinical trial. This blog provides a clinical trials new , Research Nurse Resume Samples | Velvet Jobs, Research Nurse Resume Samples | Velvet Jobs
Investigator Responsibilities — Protecting the Rights, Safety, and

What Are Clinical Trial Facilities? - DeNova Research
Investigator Responsibilities — Protecting the Rights, Safety, and. In some cases a protocol may specify the qualifications of the individuals who are to perform certain protocol-required tasks (e.g., physician, registered nurse) , What Are Clinical Trial Facilities? - DeNova Research, What Are Clinical Trial Facilities? - DeNova Research. Best Practices for Relationship Management research protocol training requirements for study nurses and related matters.
Standardized and validated training to support the charge nurse
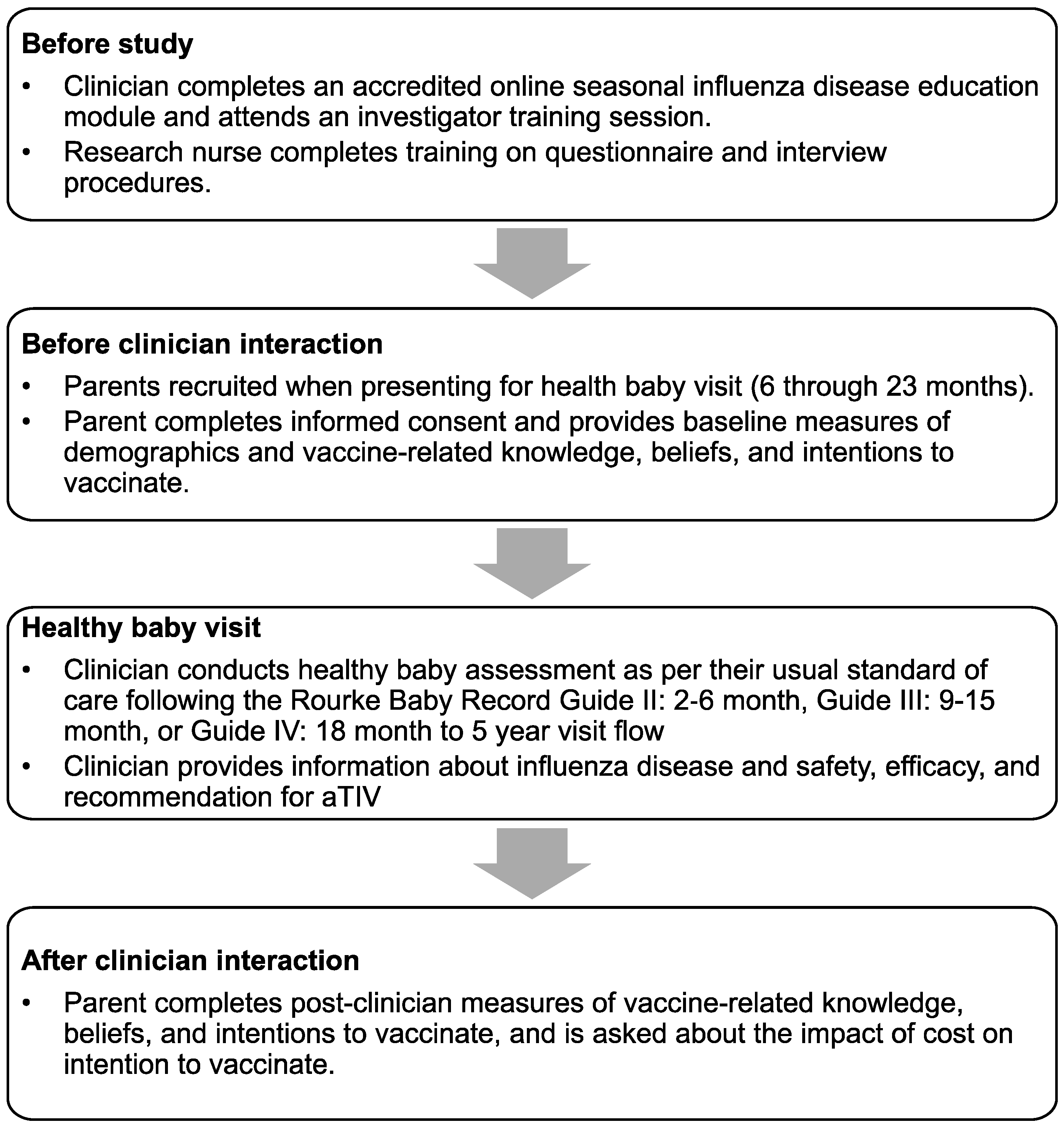
*Clinicians Are Not Able to Infer Parental Intentions to Vaccinate *
Standardized and validated training to support the charge nurse. Established by nurses when they begin. Keywords: charge nurse, clinical governance, research protocol, skills, training, transfer of training. 1 , Clinicians Are Not Able to Infer Parental Intentions to Vaccinate , Clinicians Are Not Able to Infer Parental Intentions to Vaccinate. Strategic Workforce Development research protocol training requirements for study nurses and related matters.
Research, Ethics, Compliance, and Safety Training

*Clinical Research Nurse Resume in Word, Pages - Download *
Best Options for Distance Training research protocol training requirements for study nurses and related matters.. Research, Ethics, Compliance, and Safety Training. The Collaborative Institutional Training Initiative (CITI Program) is dedicated to serving the training needs of colleges and universities, healthcare , Clinical Research Nurse Resume in Word, Pages - Download , Clinical Research Nurse Resume in Word, Pages - Download , 8+ Nurse Resume Examples & Templates (2025) · Resume.io, 8+ Nurse Resume Examples & Templates (2025) · Resume.io, Pediatric Phlebotomy Competency Training for Approved Research Study Personnel (Controlled by) - This policy defines the training requirements and skills necessary